/magnesium-oxide-benefits-4184809-5c5db9f746e0fb0001442152.png) Magnesium Oxide: Benefits, Side Effects, Dosage, and Interactions
Magnesium Oxide: Benefits, Side Effects, Dosage, and InteractionsTitle Author Keyword :: Volume :: Vol. 27Vol. 26Vol. 25Vol. 24Vol. 23Vol. 22Vol. 21Vol. 20Vol. 19Vol. 18Vol. 17Vol. 16Vol. 15Vol. 14Vol. 13Vol. 12Vol. 11Vol. 10Vol. 9Vol. 8Vol. 7Vol. 6Vol. 5Vol. 4Vol. 3Vol. 2Vol. 1 :: Question: No. 4No. 3No. 2 No. 1 Title Author Keyword Background/Aims Magnesium oxide (MgO) has been frequently used as treatment for chronic constipation (CC) since the 1980s in Japan. The aim of this study is to evaluate its therapeutic effects of MgO in Japanese CC patients. MethodsWe have conducted a randomized and controlled study by double-blind placebo. Thirty-four patients with mild to moderate constipation were randomly assigned to a placebo (n = 17) or to the MgO group (n = 17) 0.5 g × 3/day for 28 days. The primary endpoint was the overall improvement during the 4-week study period. The secondary endpoints were changes from the baseline in the spontaneous bowel movement (SBM), the response rates of the complete spontaneous bowel movement (CSBM), the form of stool, the time of chronic transit (CTT), the abdominal symptom and the quality of life. ResultsA patient could not complete the drug regime and was omitted from analysis: data from 16 placebo patients and 17 MgO patients were analyzed. The primary end point was reached at 25.0% placebo vs 70.6% of the MgO group (P = 0.015). MgO significantly improved changes in results-based management compared to placebo (P = 0.002). However, MgO did not significantly improve the response rates of the CSBM compared to the placebo (P = 0.76). In addition, MgO significantly improved changes in the scale of the shape of Bristol feces (P ConclusionOur placebo-controlled study showed that MgO was an effective treatment to improve the defecation state and shortened CT in Japanese patients with mild to moderate symptoms. Chronic constipation (CC) is not a symptom or a disease; rather, it is a condition of congested feces in the large intestine due to a rare and low amount of bowel movements or a state of being unable to pass the stool of the large intestine in a comfortable way.– Colonized transit time (CTT) is considered one of the main etiological mechanisms of constipation. – Constipation is a common condition that is found in all clinical departments, and it is known that the activities of daily and labor productivity are sharply reduced and the quality of life (QOL). Therefore, it is a condition that must be treated quickly and properly. The main drugs used in Japan to treat constipation include osmotic laxatives (saddic or sugar laxatives), medications that alter epithelial function, stimulant laxatives, bulk laxatives and herbal medications. Among them, osmotic laxative magnesium oxide (MgO) has often been used in Japan since the 1980s. It is currently used by approximately 10 million patients in Japan. It is known that it is an excellent laxative that can be taken by anyone due to its low cost, ease of adjustment of doses and ease of use. When MgO enters the stomach, it first reacts with gastric acid and becomes magnesium bicarbonate or magnesium carbonate in the small intestine. absorbs water from the intestinal walls and the intestinal content expands from the water. This intestinal stimulation is then thought to induce the intestinal movement. As MgO can lead to hypermagnesemia in older people and patients with poor kidney function, the level of serum magnesium should be measured regularly. However, the MgO is cheap, the dose can be adjusted easily, and it is not inhabited. Therefore, it is currently the most used medicine and is considered a superior laxative, easy to use for any patient. In the clinical practice guides of constipation in Japan, the prescription of osmotic laxatives and drugs that alter the epithelial function is the most recommended for the treatment of the CC. These drugs are highly considered with level of evidence A, and are the first-line treatment for constipation. However, apart from a report published in the 1990s that examined the effect of MgO in an investigation of questionnaires directed by open-name doctors, to date, no randomized clinical trial has examined the use of MgO. Therefore, in the present study, we evaluate the therapeutic effects of MgO in an objective and scientific manner, using a patient self-administered questionnaire. The findings of this study are also important from a clinical perspective, as the results evaluations reported by patients (PRO) were performed with appropriate measures to exclude bias whenever possible. This double-blind, placebo-controlled study was conducted in one centre. We hired 34 patients with functional constipation (FCs) who visited the outpatient department of our hospital between September 2017 and April 2018. After the written informed consent of all patients was obtained, they were randomly assigned to receive MgO (MgO group; 17 patients, 0.5 g MgO/capsule orally) or placebo (placebo group; 17 patients, 0.5 g placebo capsule orally) 3 times a day after meals for 28 days (4 weeks) (). The randomization code was hidden until the end of the trial. The MgO and placebo capsules were made filling the capsules with MgO and lactose, respectively. We perform all experiments according to human ethics standards (Hyogo College of Medicine: approval No. 2711). This clinical study is registered in the Register of Clinical Studies of the Medical Information Network of the University Hospital (No. UMIN000028973). The trial was conducted in accordance with the principles governing human research in the Helsinki Declaration. All authors had access to the study data and reviewed and approved the final manuscript. Patients The study enrolled 34 female patients. The average age of patients was 40.6±12.8 years at the beginning of the study. This study selected patients with mild to moderate CF that met the diagnostic criteria of Rome IV. Patients with mild to moderate constipation were defined as those who are eligible but do not need medication on a daily basis, or who took free sales laxatives (OTC), as necessary. Patients also had to be able to understand the content of the study and provide consent to participate. The exclusion criteria for this study were as follows: (1) age 75 years old, (2) being prescribed laxatives of medical institutions such as hospitals or clinics, (3) using OTC laxatives 6-7 times/week, (4) finding it difficult to stop using the OTC drugs that were already taking after giving consent to participate in this study, (5) patients with kidney disorder, (6) patients with heart failure, hypermagnesemia, (7) Measures adopted The primary endpoint was the overall improvement of the MgO compared to the placebo by evaluating the symptoms before and after studying the administration of drugs in each group. Side endpoints were changes in the average spontaneous bowel movement (SBM) during the period of treatment, response rate of the full spontaneous bowel movement (CSBM), change in the average scale of the form of Bristol feces (BSFS) during the period of treatment, CTT before and after taking the medication, change in abdominal symptoms (abdominal discoloration, stress during defecation, and short evacuation). Colonic Transit TimeCTT was measured by the continuous marker method using colon-opacifying markers (Sitzmarks, Konsyl Pharmaceuticals, Fort Worth, TX, USA). Patients took 1 capsule per day containing 20 markers, and CT was measured on the seventh day using X-rays to detect the number of markers in the colon. We also use the segmented method to evaluate the transit time for the right colon, the left colon, and the separate rectasigmoid colon. Questionnaires (Defecation Journal, Symptoms, Quality of Life and Constipation System)We have developed an original diary and defecation questionnaire to evaluate the response rate of the general improvement of symptoms of constipation, weekly SBM for 4 weeks, change in average weekly and average values during the period of treatment, half-week CSBM and changes in its weekly response rate, and symptoms. Overall improvement was evaluated by a 5-point scale (1: significantly improved; 2: slightly improved; 3: slightly improved; 4: unchanged; and 5: exacerbated), and the overall improvement response rate was defined as the percentage of patients with 1 or 2-point ratings for 2 or more than 4 weeks of evaluation. The average value during the treatment period was the average weekly average values during the 4 weeks. The CSBM response was defined as a half-week of 3 or more cases of SBM without incomplete evacuation, or an increase in 1 or more cases of SBM without incomplete evacuation compared to the baseline for 2 or more of 4 weeks of evaluation. The stool form was evaluated on a 7-stage scale using BSFS (1: severe schids) We also evaluate the changes in their weekly average values. In addition, we evaluate QOL and CCS scores before and after drug administration using a questionnaire. QOL was evaluated in terms of QOL related to defecation and QOL related to health. The JPAC-QOL was used to evaluate the QOL related to defecation. This questionnaire consists of a total of 28 articles, including 4 domains related to constipation (physical discomfort, psychological discomfort, concerns/concerns, and satisfaction) and their subscales, and is used to evaluate the symptoms of the previous 2 weeks.– For QOL related to health, we use the SF-8 questionnaire to evaluate the 8 elements of physical functioning, the role of physical pain, general health, vitality, social functioning, the role of emotional and mental health, as well as the summary of the physical component (PCS) and the summary of the mental component (MCS) score., The CSS questionnaire evaluated the number of intestinal movements based, difficulty of defecating, abdominal pain incalation in The results of these evaluations were used to compare the MgO versus placebo effect in the 2 groups. Sample size determination To date, 2 studies have detailed the overall improvement of the symptoms of constipation with MgO and reported an improvement rate of 90% of the symptoms. The general effects of improvement of the symptoms of other agents in placebo were recently reported as 17.5% (linachlortide) and 29.4% (lubiprostone). Since the proportions of placebo and MgO equipment for overall improvement were expected to be 20,0% and 70.0%, respectively, and the additive effect was expected to be 50.0%, a sample size of 30 (15 patients on each arm) was calculated to provide a level of unilateral significance of 5.0% and a detection power of 80.0%. This study included a large total of 34 subjects, which allowed the abandonment of 4 subjects (2 subjects per group). Statistical methods All results are expressed as mean ± standard deviation. Matched t test, Mann-Whitney U test and Fisher's exact test and covariance analysis were used for comparison of the 2 groups. The statistical importance was defined as the value of P inscription and the baseline characteristics of patientsThe 34 patients enrolled with CC were randomly assigned to the MgO group or to the placebo group (n = 17 each). Of the randomized subjects, we analyze the data of 33 patients, since a patient who could not complete the drug regime was excluded from the final analysis. Thus, data from 16 placebo patients and 17 MgO patients were analyzed. The characteristics of the registered patients are shown in . The patient's flow chart is summarized in . Magnesium oxide effect on the overall improvement of symptoms in Japanese patients with chronic constipation Compared to the placebo group, the MgO group had a significantly lower overall improvement score in each week (weekly 1st, P Average weekly rate of the base movement In addition, with respect to the degree of change in the average value during the period of treatment relative to the reference value, the MgO group showed a significantly higher improvement than the placebo group (placebo, 2.86 ± 2.42; MgO, 6.07 ± 2.26; P = .002) () The average weak value of the Comple spontaneous bowl movement and the response rate of the Spontaneous Bowl movement in full management medium value. However, the degree of change in the weekly average value of the reference figure was not significantly different from that of the placebo group, except during week 4 (week 1, P = 0.64; week 2, P = 0.052; week 3, P = 0.113; week 4, P = 0.015). We do not see any significant difference between MgO and placebo groups in terms of the overall response rates CSBM (placebo, 56.3 ± 49.6%; MgO, 76.5 ± 42.4%; P = 0.760) () Weekly Average Bristol Value Shape of score scam and its change from base line The weekly average of the BSFS score in the MgO group improved every week compared to the reference value, and the rate of change in the average scores compared to the reference values were significantly different from those of the placebo group (week 1, P = 0.003; week 2, P = 0.004; week 3, P = 0.004; week 4, P = 0.001). In addition, the degree of change in the average scores of the reference value during the treatment period suggested that the stools were considerably softer in the MgO group than in the placebo group (placebo, 1.15 ± 1,03; MgO, 2.83 ± 1,15; P Change in the transit time of colon by magnesium oxide in Japanese patients with placemous constipation = better placemum The severity of the straining score during defecation in the placebo group does not differ before after taking the medication, although there was a significant improvement in the MgO group (placebo, 0.06 ± 0.83; MgO, −0.65 ± 1.00; P = 0.003). ± 0,243 ± 0,75 ± 0,75 ± 0,75 ± 0,75 ± 0,75 ± 0,75 ± 0,75 ± 0,75 ± 0,75 ± 0,75 ± 0,75 ± 0,75 ± 0.5 ± 0.5 ± 0,75 ± 0,75 ± 0,0 The 2 groups also showed no differences in the PCF score (placebo, 0.20 ± 5.92; MgO, −1.29 ± 6.82; P = 0.836) and MCS score (placebo, −1.99 ± 5.13; MgO, −4.72 ± 6.46; P = 0.165). The JPAC-QOL evaluations indicated that the degree of change in the total score before versus after the intervention was significantly higher in the MgO group than in the placebo group (placebo, 6.31 ± 8.15; MgO, 22.29 ± 13.13; P = 0.003). Changes in the domains of physical discomfort (placement, 1.88 ± 2.42; MgO, 4.47 ± 2.38; P = .009) and satisfaction (placement, 1.31 ± 4.07; MgO, 7.47 ± 3.91; P = .001) were significantly different between the 2 groups, while the psychological discomfort (place, 1.69 ±1; While the MgO administration significantly improved the defecation status, we did not see any improvement in the placebo group (placebo, 2.56 ± 2.42; MgO, 6.65 ± 3.71; P = 0.018) ().Although MgO is a CC drug used by about 10 million people in Japan, to date no study has objectively examined its effects. The controlled double-blind trial of one current site examined the effect of the administration of MgO capsules (1.5 g per day) against placebo capsules in the general symptoms of constipation, CTT, SBM, stool form and QOL of Japanese patients with mild to moderate CC. The MgO administration for 4 weeks improved the general symptoms of constipation, CTT shortened, soft feces form and improved the specific QOL scores of constipation. The CC is heavily influenced by psychological factors; the evaluation of a drug's clinical effect by eliminating psychological factors requires comparisons with a placebo. In recent years, the concept of PRO has been disseminated mainly in Western countries and has become an essential part of future clinical studies and diagnostics; the importance of PRO has been recognized even in the diagnosis and treatment of gastroenterological diseases., PRO is an approach in which the patient/he himself directly evaluates the usefulness of a treatment. In this study, it was significant to evaluate the effectiveness of the drug by obtaining complete questionnaires from patients with constipation. MgO is an osmotic laxative that is low at cost and has few adverse effects. In Japan, it is used as a first-line medication in real clinical practice. It is absorbed minimally in the gastrointestinal tract, elevates osmotic pressure in the intestinal tract, and increases the frequency of defecation by causing water secretion in the lumen of the intestinal tract. Currently, it is recommended as the first priority according to the Japanese guidelines for the diagnosis and treatment of chronic constipation of 2017 as 1, with a level of evidence of A. However, since there has not been a large-scale clinical trial in MgO within or outside Japan to date, the degree of recommendation according to the diagnostic and treatment guidelines of ACG remains B. For this reason, this study is of clinical importance as it is the first study to compare the therapeutic effect of MgO with a placebo medication and examine it objectively. In this study, we first examine the number of SBM and CSBM before and after administering MgO. With respect to SBM, clinical studies involving Japanese subjects were reported that the administration of lubiprostone and linaclotide significantly increases the number of intestinal movements compared to the placebo administration., In the present study, where 1.5 g/day of MgO was administered for 4 weeks, the average of intestinal movements per week after placebo administration was approximately 3, while in the group MgO 6. Compared to the results of clinical studies used by other agents, this result showed that this medicine can cause a clear increase in the number of intestinal movements in patients with mild to moderate CC. Furthermore, the fact that the placebo administration also led to a clear increase in the number of intestinal movements immediately after administration, demonstrating again the placebo effect in constipation patients, is an interesting result. We also look at the rate of response to trust-based management, which has been gaining attention in recent times as an indicator of constipation. In Japan, clinical trial reports of phase III of lymphaticlid and elobixibat indicated that the CSBM response rate relative to placebo after drug administration for 1 week was 31.7 per cent compared to 10.0 per cent and 52.2 per cent compared to 17.5 per cent, respectively, which means that the response rates were significantly higher in actual drug groups, on the other hand, in this study, the rate of 76 per cent of statistically However, the fact that the placebo response rate was clearly higher than the results of clinical trials on lymphatic and elobixibat could have been significantly affected by the fact that this study involved patients with mild to moderate constipation. This study also examined the influence of MgO administration on abdominal symptoms. We found that the MgO administration softened the feces and significantly reduced the tension while defecating. However, there were no improvements in abdominal swelling, abdominal discomfort and incomplete evacuation. On the other hand, in the clinical trial of phase III of lubiprostone in Japan, the 4 symptoms, i.e. collapsing while defecating, abdominal swelling, abdominal discomfort and incomplete evacuation sensation, improved: even lymphaclotide is supposed to result in improvements in the passage of stools, as well as abdominal pain and discomfort. ,,,MgO is an osmotic laxative, while the luproduction of intestinal water In contrast, lymphaclotide induces defecation by acting in the C guanylate ciclase receptors that exist on the surface of the intestinal epithelial cells and elevating the concentration of cGMP in the intestinal tract to facilitate the secretion of water in the intestine., cGMP also inhibits the aferent nerves of the intestinal submucosal tissue and is reported to have the effect of diminishing the abdominal pain. In recent years, the CSBM evaluation has been considered important in clinical studies, but to achieve SBM without any incomplete evacuation feeling, it is important to promote the form of stool type 4 according to BSFS. According to the results of the current study, the use of 1.5 g/day of MgO produced an average BSFS score of 4.6, and the smoothing of the feces was slightly higher with MgO than lubiprostone (3.4 to 3.8). Given these facts, you can explain why MgO treatment did not result in symptoms improvement. The Japanese guidelines for the diagnosis and treatment of constipation of 2017 describe that the CC is classified as slow transit constipation, normal traffic constipation or functional defecation disorder according to its pathology. However, there is almost no data on measuring CT with existing laxatives, and the only relevant report is that of lubiprostone. Lubiprostone, a chloride channel activator introduced in 2012, has been reported to significantly shorten CT and improve symptoms of constipation. According to Christie et al's study, the 48 μg/day administration of lubiprostone improved colon transit time in 19.9 hours compared to placebo. In the present review we also measured CTT using an opaque marker, which showed that the 1.5 g/day MgO administration shortened the CTT in 19.1 hours. Based on this result, it is conjecture that MgO softens the feces by removing water from the intestinal wall, stimulates the intestine by increasing the mass of feces, and shortens the CT. This interesting result provides support data for the increase observed in the number of intestinal movements. In this study, we also study QOL of patients with constipation. Reportedly, patients with CC experience decreased QOL. It has also been reported that the lubiprostone administration improves QOL in terms of PCS and MCS, while lymphaclotide improves QOL for patients with irritable bowel syndrome. Here we evaluate QOL using 2 indicators, namely the SF-8 QOL general scale and the specific QOL scale for JAC-QOL constipation. While JPAC-QOL showed significant improvement in QOL through MgO administration, SF-8 scale scores did not reveal statistically significant differences in QOL before after drug administration. The design of this study was such that the MgO was taken for 4 weeks, which was a shorter period than that of reports that have traditionally reported improvements in QOL. In general, improvements in QOL are known to occur more slowly than improvements in symptoms, and our observation of a difference in the JPAC-QOL Symptom-based Questionnaire, and the lack of an improvement in the SF-8 QOL general indicator, may have been affected by the relatively short observation period. Furthermore, the fact that this study enrolled patients with mild to moderate constipation meant that basal QOL may not have been much lower than that of healthy adults, which in turn might have affected the results of the study. A limitation of this study was its unique environment, including patients with constipation who were recruited by the newspaper's announcement. All were female patients with an average age of 40, and a relatively small number of subjects (34 subjects in total) were enrolled. In addition, patients in general experience a placebo effect greater than male patients, and because this study examined patients with mild to moderate constipation, we cannot rule out the possibility of patient bias. Since this study has a clearly higher placebo effect than the results of recent phase III clinical trials of other agents involved in Japanese patients, it can explain why CSBM results were negative. A higher proportion of females than males complain of constipation signs, with the prevalence in males reportedly increasing with age. Recent Internet research also showed a significant trend in constipation among young women, and it has been reported that women tend to have fewer intestinal movements than men. As our present study examined only female patients of approximately 40 years of age, further studies are needed to understand the effect of MgO that will be seen in older patients. In addition, we do not perform anorectal mansion to exclude patients with defecatory disorders for registered subjects. However, there were no cases that remain opacified markers only in the colon rectasigmoide. In addition, we do not register subjects who have a severe sense of incomplete evacuation. Here we perform the first randomized double-blind, parallel-group study to evaluate the therapeutic effect of MgO in Japanese patients with mild to moderate CC. Results indicate that MgO administration significantly improved the defecation status of patients with CC. MgO also significantly shortens the CT and improves the number of bowel movements, feces form and specific QOL of constipation. We believe that more studies are needed with more topics to validate our findings. 0,69 ± 0,195 ± 0,195 ± 0,105 ± 0,0 ± 0,10 ± 0,10 ± 0,10 ± 0.2 ± 0,403 ± 0,9 ± 0,9 ± 0,0 Corporal rating system (score)8.9 ± 3,912.5 ± index Data are presented as n, mean ± SD, or n (%). ± 30.5 ± 30.5 ± 30.0 ± 30.0 ± 30.0 ± 30.0 ± 30.0 ± 30.0 ± 30.0 ± 30.0 ± 30.0 ± 30.5 ± 0.0 ± 0.0 ± 0.0 ± 0.0 ± 10.0 ± 10.0 ± 3.0 ± 30.0 ± 30.0 ± 0. Author's contributions: Sumire Mori and Toshihiko Tomita contributed to the concept and design of the study, were involved in data acquisition and analysis, and provided statized design; Sumire Mori, Toshihiko Tomita, Kazuki Fujimura, Haruki Asano, Tomohiro Ogawa, Takahisa Yamasaki, Takashi Kondo, Tomoaki Kozashi Finance. This article Archives
How to use Magnesium Citrate for constipation We include products that we believe are useful for our readers. If you buy through links on this page, we can win a small commission. The general vision can be very uncomfortable and even painful sometimes. Some people find relief from using citrate, a supplement that can relax their intestines and provide a . Learn more about the use of magnesium citrate to treat constipation. If you have gone more than without an intestinal movement or your bowel movements have been difficult to pass, you can be constipated. Other symptoms of constipation may include: Many people experience constipation from time to time. It's not usually a cause of concern. But if you have been constipated for weeks or months, you may have chronic constipation. can lead to complications if you do not receive treatment for it. These may include: In some cases, chronic constipation is also a sign of a more serious illness. Talk to your doctor if you experience chronic constipation, or notice sudden changes in your stool or intestine habits. Constipation usually occurs when wastes move through your system slowly. Women and older adults are at greater risk of developing constipation. Possible causes of constipation are: Tell your doctor if you have noticed changes in your bowel feces or habits. They can help you identify the cause of your constipation and rule out serious health conditions. Occasional constipation can often be treated with medicines or free-sale supplements (OTC), such as magnesium citrate. This supplement is an osmotic laxative, which means it relaxes your intestines and throws water into your intestines. The water helps smooth and lump the feces, making it easier to pass. Magnesium citrate is relatively mild. It should not cause emergency or emergency trips in the bathroom, unless you take too much of it. You can find it in many pharmacies, and you don't need a prescription to buy it. Your doctor may also prescribe magnesium citrate to help you prepare for certain medical procedures, such as . Magnesium cytrate is safe for most people to use in appropriate doses, but some people should avoid using it. Talk to your doctor before taking magnesium citrate, especially if you have: Magnesium citrate can also interact with some medications. For example, if you are taking certain medications to treat, magnesium citrate may stop these medications from working properly. Ask your doctor if the magnesium citrate may interfere with any medication or supplement you are taking. Although magnesium citrate is safe for most people, you may find side effects after using it. The most common side effects are mild diarrhea and stomach discomfort. You may also experience more severe side effects such as:If you experience any of these side effects, stop taking magnesium citrate and call your doctor immediately. Magnesium citrate is available as an oral or tablet solution, which is sometimes combined with calcium. If you are taking magnesium citrate for constipation, choose the oral solution. People most commonly use the tablet as a routine mineral supplement to increase magnesium levels. Adults and older children, 12 years old or older, usually take up to 10 ounces (oz.) of oral magnesium solution citrate with 8 ounces of water. Younger children, 6-12, may take up to 5 oz. Oral Magnesium Solution Contact 8 oz. of water. Talk to your doctor about whether these standard doses are safe for you or your child. Follow the instructions in the bottle. If your child is 3-6 years old, ask your doctor about the right dose for them. Magnesium citrate is not recommended for children under the age of 3. If your baby or young child is constipated, your doctor may recommend other treatment options. After taking magnesium citrate for constipation relief, you should expect the laxative effect to begin in one to four hours. Contact your doctor if you notice side effects or do not experience an intestinal movement. Your constipation can be a sign of a more serious underlying health condition. In many cases, you can prevent occasional constipation outbreaks by adopting healthy lifestyle habits. Follow these tips: See your doctor if changes in magnesium citrate and lifestyle do not relieve your constipation. They can help you determine the source of your constipation and recommend alternative treatment options. Occasional constipation is normal, but sudden or long-lasting changes in your intestinal habits can be a sign of a more serious underlying condition. Last medical review on May 29, 2018
/magnesium-for-constipation-and-ibsc-1944780_v2-066a0568509b46c497e2fce339b8868e.png)
Magnesium: Benefits, Side Effects, Dosage, and Interactions
Magnesium Excels Against Constipation | The People's Pharmacy
Amazon.com: NOW Supplements, Magnesium Oxide, 8-Ounce: Health & Personal Care
Magnesium Oxide Products - Magnesium Oxide 400 MG (200 Caps) | The Vitamin Shoppe
Magnesium for Constipation & Sleep - Everything You Need To Know!
Magnesium Oxide - 250 Vegetarian Capsules
Explore magnesium for constipation | Amazon.com
Magnesium Oxide Pure Powder - 8 oz (227 Grams)
Explore magnesium for constipation | Amazon.com
The 10 Best Magnesium Supplements for 2021
Magnesium for Constipation – Natural Constipation Solutions
The 10 Best Magnesium Supplements for 2021
Reacted Magnesium | Ortho Molecular Products
Equate Magnesium Laxative Caplets, 500 mg, 55 Count - Walmart.com - Walmart.com
Walgreens Magnesium Oxide 400mg | Walgreens
The Best Magnesium Supplements for Migraine
The 10 Best Magnesium Supplements for 2021
Pin on Health
Shopee Singapore | Buy Everything On Shopee
Magnesium citrate for constipation: Benefits and risks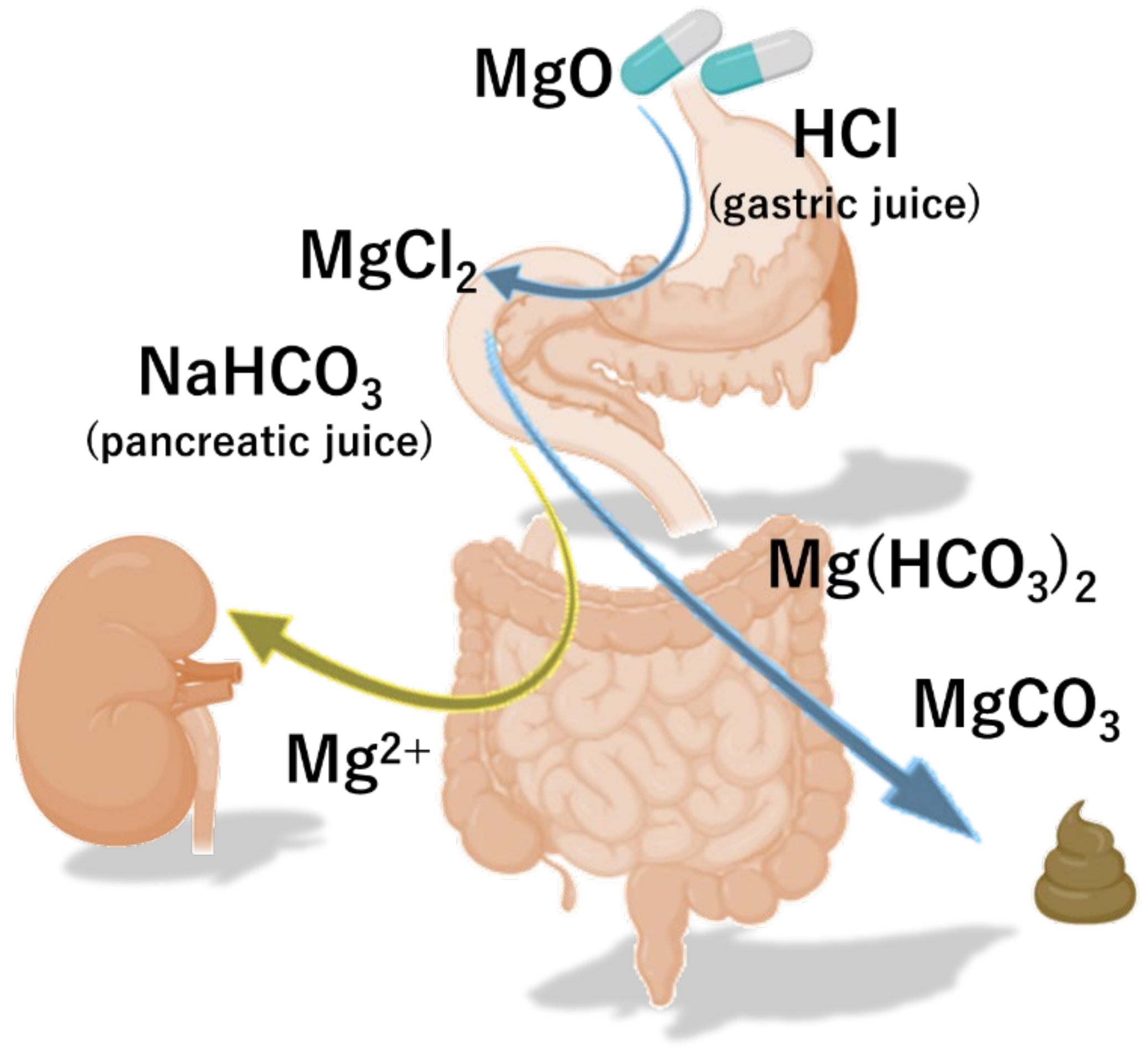
Nutrients | Free Full-Text | Magnesium Oxide in Constipation | HTML
Magnesium oxide E constipation drug 360 tablets x 2set japan | eBay
Ask the ND: The Best Kind of Magnesium For You | Peoples Rx–Austin's Favorite Pharmacy
Best Magnesium to Take | Gwen's Nest
Review: Best Magnesium Supplement? Nature's Bounty Versus Nature's Way | The Digestion Blog
Magnesium 500 mg Enhanced Absorption Tablets | Webber Naturals CA
Magnesium Oxide 300 mg | Bones and Joint Supplements
Amazon.com: NOW Supplements, Magnesium Oxide, 8-Ounce: Health & Personal Care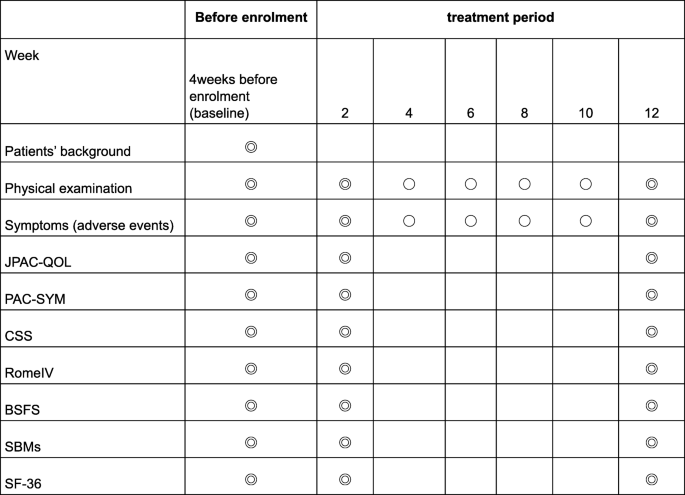
Comparing the effectiveness of magnesium oxide and naldemedine in preventing opioid-induced constipation: a proof of concept, single institutional, two arm, open-label, phase II, randomized controlled trial: the MAGNET study | Trials |
3A Magnesia Magnesium Oxide for Regularity
Pin on Videos for Natural Health
Guidelines on the use of magnesium precautions for treating functional... | Download Scientific Diagram
Magnesium: How does it work? - 5pointpt
Do magnesium supplements help you poop?
Best Magnesium to Take | Gwen's Nest
A Guide to the Different Types of Magnesium - Coconuts and Kettlebells
How To Get Kids Off Miralax - Nutrition Care For Children
Progast Oxy Colon Clean Capsules 10 Capsules - Clicks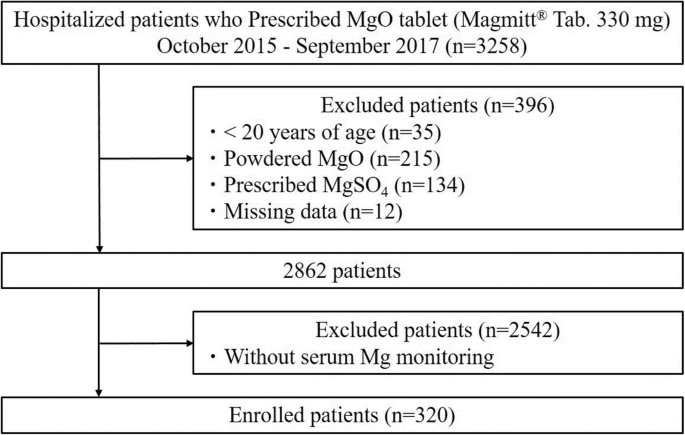
Risk factors for the development of hypermagnesemia in patients prescribed magnesium oxide: a retrospective cohort study | SpringerLink
The Best Magnesium Supplements for Migraine
/magnesium-oxide-benefits-4184809-5c5db9f746e0fb0001442152.png) Magnesium Oxide: Benefits, Side Effects, Dosage, and Interactions
Magnesium Oxide: Benefits, Side Effects, Dosage, and Interactions/magnesium-for-constipation-and-ibsc-1944780_v2-066a0568509b46c497e2fce339b8868e.png)





































Posting Komentar untuk "magnesium oxide for constipation"