 Nicotinamide - an overview | ScienceDirect Topics
Nicotinamide - an overview | ScienceDirect TopicsNiacin Niacin Names Pronunciation Piridina-3-carboxylic acid Other names Identifiers 3D model () 3DMet 109591 3340 CID (EPA) Properties C6H5NO2 123.111 g·mol−1 Appearance White crystals, translucent 1,473 grams -3 237 °C; 458 °F; 510 K 18 g L-1 0.219 (pKa) 2.0, 4.85 4.75 (nD) 1.4936 0.1271305813 D Thermochemical (ΔfH)298) −344.9 kJ mol-1 (ΔcH)298) −2.73083 MJ mol−1 Pharmacology () () () () Intramuscular, by mouth : 20 to 45 min Dangerous Warning H319 P264, P280, P305+351+338, P337+313, P501 (Pending fire) 193 °C (379 °F; 466 K) 365 °C (689 °F; 638 K) Except where otherwise noted, data are given for materials in its (at 25 °C [77 °F], 100 kPa). And? WarningNFPA 704NiacinINN: Nicotynic acid Clinical dataNiacor, Niaspan, others/ Legal status Identifiers (EPA) Niacin, also known as nicotynic acid, is a form and a form of , a . It can be manufactured by plants and animals of amino acid. Niacin is obtained in the diet of a variety of and , with greater content in , meat, poultry, red fish like and , smaller amounts in nuts, legumes and seeds. Niacin as used to treat, a disease caused by niacin deficiency. Signs and symptoms of the skin include skin and mouth injuries, anemia, headaches and tiredness. Many countries demand their addition to wheat flour or others, thereby reducing the risk of hair. Niacinnicotynic acid The derivative of the amide (niacinamide) is a component of the coenzymes (NAD) and (NADP+). Although niacin and (niacinamide) are identical in their vitamine activity, nicotinamide does not have the same pharmacological effects, or side effects as niacin, that is, when niacin takes in the group of -amide, it does not reduce cholesterol or cause. Nicotinamide is recommended as a treatment for niacin deficiency because it can be given in corrective amounts without causing breakage, considered an adverse effect. Niacin is also a prescription drug. The excessive amount of dietary intake recommended for vitamin functions will lower the blood and (LDL-C), and increase the blood (HDL-C, often known as "good" cholesterol. There are two forms: niacin of immediate and sustained liberation. Initial prescription amounts are 500 mg/day, increased over time until a therapeutic effect is achieved. Immediate release doses can be as high as 3,000 mg/day; sustained release up to 2,000 mg/day. Despite the changes in lipids tested, niacin has not been found to be useful in reducing the risk of those already in a . A 2010 review had concluded that niacin was effective as monotherapy, but a 2017 review incorporating double-tests concluded that the prescribed niacin, while affecting lipid levels, did not reduce mortality for all causes, or fatal cardiovascular mortality, the prescribed niacin. Niacin of prescription was caused by hepatotoxicity and increased risk of . Niacin recipes in the United States had peaked in 2009, at 9.4 million, declining to 1.3 million by 2017. Niacin has C6H5NO2 and belongs to the group of . As for (NAD) and (NADP), niacin is involved in DNA repair. ContentDefinition[] Nicin is a vitamin, that is, an essential nutrient, marketed as a dietary supplement, and in the USA, a prescription drug. As a vitamin, it is a precursor of coenzymes (NAD) and (NADP). These compounds are coenzymes for many dehydrogens, participating in many hydrogen transfer processes. NAD is important in fat, carbohydrates, proteins and alcohol, as well as cell signaling and DNA repair, and NAPA mainly in reactions such as fatty acid and cholesterol synthesis. The recommendations of vitamin consumption made by several countries are that intakes from 14 to 18 mg/day are sufficient to meet the needs of healthy adults. Niacin or (niacinamide) are used for the prevention and treatment of , a disease caused by lack of vitamin. When niacin is used as a medication to treat, daily doses vary from 500 to 3,000 mg/day. High-dose nicotinamide does not have this medicinal effect. Vitamin deficiency[] The severe deficiency of niacin in the diet causes the disease, characterized by , sensitive to the sun that involves hyperpigmentation and thickening of the skin (see image), swelling of the mouth and tongue, delirium, dementia and if not treated, death. Common psychiatric symptoms include irritability, poor concentration, anxiety, fatigue, memory loss, restlessness, apathy and depression. Biochemical mechanisms for neurodegeneration caused by observed deficiency are not well understood, but may rest in: A) the requirement of (NAD+) to suppress the creation of neurotoxic metabolites of tryptophan, B) inhibition of the generation of mitochondrial ATP, which results in cellular damage; C), activation of the pathway (PARP), as PARP is a nuclear enzyme involved in repairing the pathogenic acid. Niacin deficiency is rarely observed in developed countries, and is more typically associated with poverty, malnutrition or chronic secondary malnutrition. It also tends to occur in less developed areas where people eat (corn) as basic food, as corn is the only low grain in digestible niacin. A cooking technique called, i.e., pretracting with alkaline ingredients, increases the bioavailability of niacin during the production of corn/flor. For this reason, people who consume corn like tortillas or have less risk of niacin deficiency. To address the deficiency, the World Health Organization (WHO) recommends the management of niacinamide instead of niacin, to avoid the side effect commonly produced by niacin. Guidelines suggest using 300 mg/day for three to four weeks. Dementia and dermatitis show improvement within a week. Because other vitamin B deficiencies may be present, WHO recommends a multivitamin in addition to niacinamide. is a nutritional disorder that results in niacin deficiency. It is called after an English family with a genetic disorder that resulted in an inability to absorb the essential amino acid, tryptophan being a precursor to niacin synthesis. Symptoms are similar to hair, including red rash, squamous and sensitivity to sunlight. Oral niacin or niacinamide is given as treatment for this condition in doses ranging from 50 to 100 mg twice a day, with a good prognosis if identified and treated early. Niacin synthesis is also poor in , due to metabolic deviation in its form. Measure the state of vitamins[] The plasma concentrations of niacin and niacin metabolites are not useful markers of the niacin state. The urinary excretion of the methylated metabolite N1-methyl-nicotinamide is considered reliable and sensitive. Measurement requires a 24-hour urine collection. For adults, a value of less than 5.8 μmol/day represents the deficient niacin state and 5.8 to 17.5 μmol/day represents low. According to the World Health Organization, an alternative way of expressing urinary N1-methyl-nicotiny is like mg/g of creatinine in a 24-hour urine collection, with a deficiency defined as 4.3 Niacin deficiency occurs before the signs and symptoms of the pellagra appear. Concentrations of rythrocyte (NAD) may provide another sensitive indicator of niacin depletion, although poor, low and adequate definitions have not been established. Finally, plasma decreases in a low niacin diet because tryptophan becomes niacin. However, low tryptophan could also be caused by a low diet in this essential, so it is not specific to confirm the state of vitamin. Dietary recommendations[] Dietary recommendations Australia and New Zealand Age group RDI for niacin (mg NE/day) Higher level of consumption Children 0-6 months 2 mg/d preformed niacin* ND Children 7-12 months 4 mg/d NE* 1-3 6 10 4-8 8 15 9-13 12 20 14-18 - 30 19+ - 35 Women 14+ 14 - Men 14+ 16 Pregnant women 14–50 18 - Pregnant women 14-18 - 30 Pregnant women 19–50 - 35 Infant women 14–50 17 - Breastfeeding women 14-18 - 30 Breastfeeding women 19–50 - 35 ♪ Ingestion suitable for babies Canada Age group (years) Niacin RDA (mg NE/d) tolerable upper intake level 0-6 months 2 mg/d preformed niacin* ND 7-12 months 4 mg/d NE* 1-3 6 10 4-8 8 15 9-13 12 20 Women 14-18 14 30 Men 14-18 16 Women 19+ 14 35 Men 19+ 16 Pregnant women 18 30 Pregnant women 18-50 18 35 Infant women 17 30 Breastfeeding women 18-50 17 35 European Food Safety Authority Gender Appropriate Ingestion (mg NE/MJ) Women 1.3 Men 1.6 Age (years) tolerable upper limit of nicotynic acid (mg/day) tolerable superior limit of Nicotinamide (mg/day) 1-3 2 150 4-6 3 220 7-10 4 350 11-14 6 500 15-17 8 700 United States Age group RDA for niacin (mg NE/day) tolerable upper intake level Children 0-6 months 2* ND** Children 6-12 months 4* 1-3 6 10 4-8 8 15 9-13 12 20 Women 14-18 14 30 Men 14-18 16 30 Women 19+ 14 35 Men 19+ 16 35 Pregnant women 14-18 18 30 Pregnant women 19–50 18 35 Breastfeeding women 14-18 17 30 Breastfeeding women 19–50 17 35 * Intake appropriate for infants, as a GDR has not yet been established** It is not possible to establish; the source of consumption must be formula and food only Australia and New Zealand Age group RDI for niacin (mg NE/day) Higher level of consumption Children 0-6 months 2 mg/d preformed niacin* ND Children 7-12 months 4 mg/d NE* 1-3 6 10 4-8 8 15 9-13 12 20 14-18 - 30 19+ - 35 Women 14+ 14 - Men 14+ 16 Pregnant women 14–50 18 - Pregnant women 14-18 - 30 Pregnant women 19–50 - 35 Infant women 14–50 17 - Breastfeeding women 14-18 - 30 Breastfeeding women 19–50 - 35 ♪ Ingestion suitable for babies Canada Age group (years) Niacin RDA (mg NE/d) tolerable upper intake level 0-6 months 2 mg/d preformed niacin* ND 7-12 months 4 mg/d NE* 1-3 6 10 4-8 8 15 9-13 12 20 Women 14-18 14 30 Men 14-18 16 Women 19+ 14 35 Men 19+ 16 Pregnant women 18 30 Pregnant women 18-50 18 35 Infant women 17 30 Breastfeeding women 18-50 17 35 European Food Safety Authority Gender Appropriate Ingestion (mg NE/MJ) Women 1.3 Men 1.6 Age (years) tolerable upper limit of nicotynic acid (mg/day) tolerable superior limit of Nicotinamide (mg/day) 1-3 2 150 4-6 3 220 7-10 4 350 11-14 6 500 15-17 8 700 United States Age group RDA for niacin (mg NE/day) tolerable upper intake level Children 0-6 months 2* ND** Children 6-12 months 4* 1-3 6 10 4-8 8 15 9-13 12 20 Women 14-18 14 30 Men 14-18 16 30 Women 19+ 14 35 Men 19+ 16 35 Pregnant women 14-18 18 30 Pregnant women 19–50 18 35 Breastfeeding women 14-18 17 30 Breastfeeding women 19–50 17 35 * Intake appropriate for infants, as a GDR has not yet been established** It is not possible to establish; the source of consumption must be formula and food only The U.S. Institute of Medicine (meeting in 2015) updated Estimated Average Requirements (YEARs) and Recommended Dietary Allowances (DAA) for Niacin in 1998, also (ULs). Instead of a GDR, appropriate intakes (AI) are identified for populations for which there is insufficient evidence to identify a sufficient dietary intake level to meet the nutrient needs of most people. (see table). The (EFSA) refers to the collective set of information such as Dietary Reference Values (DRV), with the intake of Population Reference (PRI) instead of RDA, and Media Requisition instead of EAR. For the EU, AIs and ULs have the same definition as in the United States, except that the units are milligrams per megajoule (MJ) of energy consumed instead of mg/day. For women (including pregnant or nursing), men and children the PRI is 1.6 mg per megajoule. Since conversion is 1 MJ = 239 kcal, an adult who consumes 2390 kilocalories should consume 16 mg of niacin. This is comparable to GDRs (14 mg/day for adult women, 16 mg/day for adult men). ULs are established by identifying amounts of vitamins and minerals that cause adverse effects, and then by selecting as a higher limit amounts that are the "maximum daily intake unlikely to cause adverse health effects." Regulatory agencies in different countries do not always agree. For the United States, 30 or 35 mg for adolescents and adults, less for children. The UL EFSA for adults is set to 10 mg/day - about a third of the American value. For all government ULs, the term applies to niacin as a supplement consumed as a dose, and is intended as a limit to avoid skin reaction. This explains why for EFSA, the recommended daily intake can be higher than UL. Both DRI and DRV describe the necessary amounts as niacin equivalents (NE), calculated as 1 mg NE = 1 mg niacin or 60 mg of the essential amino acid tryptophan. This is because amino acid is used to synthesize vitamin. For the purposes of labelling food and dietary supplements in the United States, the amount in a portion is expressed as a percentage of (%DV). For niacin labeling purposes 100% of the daily value is 16 mg. Before May 27, 2016, 20 mgs were checked to match the GDR. By 1 January 2020, compliance with updated labelling standards was necessary for manufacturers of 10 million or more in annual food sales, and by 1 January 2021 for manufacturers with lower volume food sales. A table of the old and new adult daily values is provided in . Sources[] The nicin is found in a variety of and , including , from various animal sources , and . In general, foods with animal origin provide about 5-10 mg of niacin per serving, although dairy foods and eggs have little. Some foods of vegetable origin, such as nuts and grains, provide approximately 2-5 mg of niacin per serving, although this naturally present niacin is largely linked to polysaccharides and glycopepides, which makes it only about 30% bioavailable. Fortified food ingredients such as wheat flour have added niacin, which is bioavailable. Among the entire sources of foods with the highest content of niacin per 100 grams: Source Amount (mg / 100g) Serr = 2 Tbsp (16 g) contains 56 mg 350 , yellowfin 22.1 14.3 13.1 10.4 , light, canned 10.1 10.0 depending on which part, how cooked 7-12 depending on which part, how cooked 7-12 Source Amount (mg / 100g) depending on which part, how cooked 4-8 depending on which part, how cooked 4-8 7.0 , white, canned 5.8 3.6 , white 3.6 2.5 2.5 2.0 Source Amount (mg / 100g) 1.7 , baked, with skin 1.4 (Maíz) 1.0 0.5 0.4 0.1 0.1 0.1 0.1 and diets can provide adequate amounts if products such as nutritional yeast, peanuts, peanut butter, tahini, brown rice, mushrooms, avocado and sunflower seeds are included. Fortified foods and dietary supplements can also be consumed to ensure proper intake. Food preparation[] Naturally found nicin in foods is susceptible to the destruction of high heat cooking, especially in the presence of foods and acid sauces. It is water-soluble, and can also be lost from boiled food in water. Food fortification[] Countries fortify nutrient food to address known deficiencies. As of 2020, 54 countries needed fortification of wheat flour with niacin or niacinamide; 14 also ordered fortification of corn flour and 6 rice fortification mandate. From country to country, niacin fortification varies from 1.3 to 6.0 mg/100 g. As a dietary supplement[] In the United States, niacin is sold as a nonprescription dietary supplement with a range of 100 to 1000 mg per serving. These products often have a Structure/Function Health Claim allowed by the United States Medicines Administration (FDA). An example would be "It provides a healthy blood lipid profile." The American Heart Association strongly recommends against the replacement of niacin dietary supplement for prescription niacin due to potentially serious side effects, which means that niacin should only be used under the supervision of a health professional, and due to the manufacture of niacin dietary supplement is not as well regulated by the FDA as prescribed niacin. More than 30 mg of niacin consumed as a dietary supplement can cause skin washing. Face, arms and chest skin becomes red due to vasodilation of small subcutaneous blood vessels, accompanied by heat, tingling and itching sensations. These signs and symptoms are usually transient, from minutes to hours; they are considered unpleasant instead of toxic. As a lipid-modifying medicine[ ] In the United States, prescribed niacin, in forms of immediate release and slow release, is used to treat primary and . It is used either as monotherapy or in combination with other lipid modifiers. The doses begin at 500 mg/day and are often gradually increased to 3000 mg/day for immediate release or 2000 mg/day for slow release (also known as sustained release) to achieve specific lipid changes (low LDL-C and triglycerides, and higher HDL-C). The requirements in the United States peaked in 2009, at 9.4 million and had been reduced to 1.3 million by 2017. By the end of 2017, Avondale, having acquired the rights to Niacor of Upsher Smith, increased the price of the drug by more than 800%. Systematic revisions did not find any effect of prescribed niacin on mortality due to all causes, cardiovascular mortality, myocardial infarction, or fatal or non-mortal strokes despite cholesterol elevation. Notified side effects include increased risk of type 2 diabetes. Mechanisms[] Nicin reduces the synthesis of low-density lipoprotein cholesterol (LDL-C), low-density lipoprotein cholesterol (VLDL-C), and increases cholesterol (HDL-C). Lipidic-therapeutic effects of niacin are partly mediated through activation of , including (HCA2) and (HCA3), which are highly expressed in . HCA2 and HCA3 inhibit production (cAMP) and thus suppress free body fat release (FFAs), reducing their availability to the liver to synthesize circulating blood lipids. A decrease in free fatty acids also suppresses the liver expression of and, thus increasing the rotation of VLDL-C and reducing its production. Niacin also directly inhibits the action of (DGAT2) a key enzyme for triglycerides synthesis. The mechanism behind the niacin that increases HDL-C is not fully understood, but seems to occur in several ways. Niacin increases levels by inhibiting the breakdown of this protein, which is a component of HDL-C. It also inhibits HDL-C liver absorption by suppressing gene production (CETP). Stimulate monocytes and macrophages and, resulting in the transport of inverse cholesterol. Combined with statins[]Advanced release niacin was combined with the commercial name, and with , commercial name as combinations of prescription drugs. Advicor was approved by the United States Food and Drug Administration in 2001. Simcor was approved in 2008. Subsequently, the major test results using these niacin and statin therapies could not prove the incremental benefit of niacin beyond statin therapy. The FDA withdrew the approval of both drugs in 2016. The reason given: "Based on the collective evidence of several major cardiovascular trials, the Agency has concluded that the entire scientific evidence no longer supports the conclusion that a reduction induced by the drug at triglyceride levels and/or increase in HDL-cholesterol levels in patients treated with statins results in a reduction in the risk of cardiovascular events." The drug company suspended the drugs. Contraindications[]Immediate release (Niacor) and prolonged liberation (Niaspan) niacina are for people with active or past history because both, but especially Niaspan, have been associated with cases of serious, sometimes fatal, liver failure. Both products are contraindicated for people with existing bleeding problems, or other bleeding problems because niacin lowers the platelet count and interferes with blood clotting. Both products are also contraindicated for pregnant women or expect to become pregnant because safety during pregnancy has not been evaluated in human trials. These products are contraindicated for women who are breast-feeding because niacin is known to be excreted in human milk, but the amount and potential of adverse effects on the infant is not known. Women are advised not to sicken their child or stop using the drug. High-dose niacin has not been tested or approved for use in children under the age of 16. Adverse effects[ ] The most common adverse effects of medicinal niacin (500-3000 mg) are washed (e.g., heat, redness, itching or tingling) of the face, neck and chest, headache, abdominal pain, diarrhea, nausea, vomiting, rash. This can be minimized by starting low-dose therapy, gradually increasing the dose and avoiding administration with an empty stomach. Acute adverse effects of high-dose niacin therapy (1-3 grams per day) – which is commonly used in the treatment of – may include more, fatigue, and , acidity, blurred or impaired vision, and . With long-term use, the adverse effects of high-dose niacin therapy (750 mg per day) also include (associated with fatigue, nausea and ), and ; these hepatotoxic effects of niacin occur more often when prolonged release is used. The long-term use of niacin to greater or equal to 2 grams per day also significantly increases that of , , and , , , and diarrhea. Flushing[] – in the short term of the skin, causing the color of the reddish skin – usually lasts about 15 to 30 minutes, although it can sometimes persist for weeks. Typically, the face is affected, but the reaction may extend to the neck and upper chest. The cause is dilation of blood vessels due to elevation in GD2 () and . It was often thought that flushing involved histamine, but it has been shown that histamine did not participate in the reaction. Sometimes the rinse is accompanied by a sensation or a puncture, in particular, in areas covered by clothing. Prevention of waste requires disrupting or blocking the mediated pathway by prostaglandin. Take half an hour before the niacin prevents the breakage, as it does. Taking niacin with meals also helps to reduce this side effect. Acquired tolerance will also help reduce waste; after several weeks of a consistent dose, most people no longer experience waste. Slow or "sustained" forms of niacin have been developed to decrease these side effects. Liver damage[] Nicin in medicinal doses can cause modest serum and non-binding elevations, both liver lesion biomarkers. Increases generally resolve even when drug intake continues. However, less commonly, the form of sustained release of the drug can lead to gravity, with the beginning in days to weeks. Early symptoms of severe liver damage include nausea, vomiting, and abdominal pain, followed and . The mechanism is believed to be a direct toxicity of high serum niacin. Lowering the dose or changing to the immediate release form can resolve the symptoms. Rarely, the injury is severe, and progresses to liver failure. Diabetes[ ]The high doses of niacin used to treat hyperlipidemia have been shown to rise in people with type 2 . Long-term niacin therapy was also associated with increased risk of type 2 diabetes. Other adverse effects[] High doses of niacin can also cause niacin, a thickening of the and , leading to blurred vision and blindness. This maculopathy is reversible after the intake of niacin ceases. Niaspan, the slow-release product, has been associated with a reduction in platelet content and a modest increase in protrombin time. Pharmacology[]Pharmacodynamics[] Nicotinamide and nicotinamide become the NAD. NAD converts NADP by phosphorylation in the presence of the enzyme. NAD and NADP are coenzymes for many, participating in many hydrogen transfer processes. NAD is important in fat catabolism, carbohydrates, proteins and alcohol, as well as cell signaling and DNA repair, and NAPA mainly in anabolistic reactions such as fatty acid and cholesterol synthesis. High energy needs (brain) or high rotational organs (gut, skin) are usually the most susceptible to their deficiency. The activation of HCA2 has effects other than reducing serum cholesterol and triglyceride concentrations: antioxidative, anti-inflammatory, antitrombotic, improving function and stability, all of which contradicts the development and progression of atherosclerosis. Niacin enzymes, and . Niacin produces a serum increase in normal individuals and those with . However, in the Gilbert Syndrome, the increase in bilirubin is greater and the cleaning is delayed more than in normal people. A test used to help diagnose Gilbert's syndrome involves intravenous nicotynic acid (niacin) administration at a dose of 50 mg during a period of 30 seconds. Pharmacokinetics[] Both niacin and niacinamide are quickly absorbed from the stomach and small intestine. Absorption is facilitated by sodium-dependent diffusion, and higher intakes, through passive diffusion. Unlike some other vitamins, the absorbed percentage does not decrease with the increasing dose, so even in amounts of 3-4 grams, the absorption is almost complete. With a dose of a gram, maximum plasma concentrations are reached from 15 to 30 μg/mL within 30 to 60 minutes. Approximately 88% of an oral pharmacological dose is eliminated by the kidneys as an unchanged niacin or nicotinuric acid, its primary metabolite. Half of the life of plasma-deleted niacin varies from 20 to 45 minutes. Niacinamide is the main form in the bloodstream. In the liver, niacinamide becomes storage (NAD). As necessary, the NAD of the liver is hydrolysed to niacinamide and niacin for transport to tissues, reverted to the NAD to serve as the cofactor of enzymes. Excess niacin is methylated in the liver to N1-methylnicotinamide (NMN) and excreted in the urine as such or as the N1-methyl-2-piridone-5-carboxamide metabolite (2-piridone). The decrease in urinary content of these metabolites is a measure of niacin deficiency. Production[]Biosyntesis[]In addition to absorbing niacin from the diet, niacin can be synthesized from the essential, a five-step process with the penultimate compound (see figure). Some bacteria and plants use in a path that also goes to quinolinic acid. For humans, conversion efficiency is estimated to require 60 tryptophan to make 1 mg of niacin. and are necessary for the process. Pellagra is a consequence of a diet that dominates corn because niacin in corn is poorly bioavailable and corn proteins are low in tryptophan compared to wheat and rice proteins. Industrial synthesis[] occurs by ammoxidation of . is then used to catalyze nicotinonitrile to nicotinamide, which can be converted to niacin. Alternatively, ammonia, acetic acid and paraldehide are used to make 5-ethyl-2-methyl-piridine, which then becomes directly niacin. The demand for commercial production includes animal feed and food fortification for human consumption. According to , 31,000 tons of nicotinamide were sold worldwide in 2014. Chemistry[] This colourless water-soluble solid is a derivative of , with a (COOH) in the 3-position. Other forms of vitamin B3 include the corresponding (niacinamide), where the carboxyl group has been replaced by a group (CONH2). Preparations[] The nicin is incorporated in multivitamin and is sold as a dietary supplement of a single ingredient. The latter may be of immediate or slow release. (niacinamide) is used to treat niacin deficiency because it does not cause the adverse reaction of niacin view washing. Nicotinamide can be toxic to the liver in doses above 3 g/day for adults. Prescription products can be immediate release (Niacor, 500 mg tablets) or (Niaspan tablets, 500 and 1000 mg). Niaspan has a film coating that delays the release of niacin, which results in an absorption during a period of 8 to 12 hours. This reduces and affects side effects, but increases the risk of comparison with immediate release medication. Prescription niacin in combination with statin medications (continues) is described above. A combination of niacin and had been approved for use in Europe and marketed as Tredaptive. Laropiprant is a binding medication that shows reducing niacin-induced vasodilation and the side effects of washing. A clinical trial did not show extra efficacy of Tredaptive in cholesterol reduction when used along with other statin drugs, but showed an increase in other side effects. The study led to the withdrawal of Tredaptive from the international market. A form of dietary supplement sold in the USA is hexanicotin inositol (IHN), also called . This has been with niacin in the six inositol alcohol groups. IHN is usually sold as "free" or "not fluid" niacin in units of 250, 500, or 1000 mg/tes or capsules. In the United States, it is sold as a free sales formulation, and is often marketed and labeled as niacin, so deceptive consumers think they are getting an active form of medication. While this form of niacin does not cause breakage associated with immediate release products, there is not enough evidence to recommend IHN to treat hyperlipidemia. History[] Nicin as a chemical compound was first described by chemistry in 1873 in its studies, but which for many years predated the concept of food components other than proteins, fats and carbohydrates that were essential to life. The nomenclature of vitamins was initially alphabetic, with calling these A soluble in fat and B soluble in water. Over time, eight chemically distinct, water-soluble vitamins B were isolated and numbered, with niacin as vitamin B3. Maize (maize) became a basic food in the southeast of the United States and parts of Europe. A disease that was characterized by skin dermatitis exposed by sunlight was described in Spain in 1735 by . It attributed the cause to poor diet. In northern Italy it was called "pellagra" of the (agra = -like or -like; pell = skin). Over time, the disease was more closely linked specifically to corn. In the United States, he was assigned to study pellagra by the United States' General Surgeon. His studies confirmed a diet based on corn as the culprit, but did not identify the root cause. Nicotynic acid was extracted from the liver by biochemical in 1937. He later identified the active ingredient, referring to it as "prevention factor of the pest" and the "anti-black factor". It is also known as "PPvitamine", "P-P-vitamin" and "PPP factor", all derived from the term "preventive-pellagrating factor". By the end of the 1930s, the studies of , Marion Blankenhorn, and Clark Cooper confirmed that niacina cured the hair in humans. The prevalence of the disease was considerably reduced as a result. In 1942, when the flour began with nicotynic acid, a popular press headline said "Tobacco in Your Bread". In response, the Food and Nutrition Council of new niacin and amida niacina names for use mainly by non-scientists. It was thought appropriate to choose a name to dissociate nicotynic acid from , to avoid the perception that vitamins or foods rich in niacin contain nicotine, or that cigarettes contain vitamins. The resulting name niacin is derived from nicotynic acid + vitamin. Carpenter found in 1951, that niacin in corn is not biologically available, and can be released only in very alkaline water of 11. This explains why a Latin American culture that used the alkaline treated cornalin to make the tortilla was not at risk of niacin deficiency. In 1955, Altschul and his colleagues described large amounts of niacin that had a low lipid property. As such, niacin is the oldest known . , the first drug '', was first marketed in 1987. Research[]In and , niacin produces anti-inflammatory effects marked in a variety of tissues, including the brain, gastrointestinal tract, skin, and through activation of (HCA2), also known as niacin receptor 1 (NIACR1). Unlike niacin, nicotinamide does not activate NIACR1; however, both niacin and nicotinamide activate the in vitro (GPER). ########################################################################################################################################################################################################################################################## Water soluble Combinations () Niacin and derivatives Other peripheral vasodilators (), / () (, ) Niacin and derivatives ( and ) inhibitors () inhibitors () () () () Combinations Other , / () (, ) Niacin and derivatives ( and ) inhibitors () inhibitors () () () () Agonists Mixed() Antagonists Agonists Antagonists Unknown See also Agonists Mixed() Antagonists Agonists Antagonists Unknown constituents / Other/non-assorted See also: • See also: Navigation menu Personal tools Named spaces Variants Views More Search Navigation Contributed Tools Printing/exporting Other projects Languages
Accessibility links Search results Niacin - Wikipedia Therapy Vitamin B-3 (Niacina)Vitamin B3 or Nicotinic Acid Importance TENID Vitamin ... - YouTubeVitamin B3 (Niacina): Brands, Medical Use, Clinical DataVitamin B3 or Nicotynic Acid Sources Food confidentiality vitamin b3 rich ...Vitamin B3 Supplements / Niacin ...

Nicotinamide Derivative - an overview | ScienceDirect Topics
Nicotinate Phosphoribosyltransferase - an overview | ScienceDirect Topics
Niacin - Wikipedia
Nicotinic Acid - an overview | ScienceDirect Topics
Nicotinate Phosphoribosyltransferase - an overview | ScienceDirect Topics
Nicotinic Acid - an overview | ScienceDirect Topics
Pyridoxal 5 -Phosphate-Dependent Enzymes at the Crossroads of Host–Microbe Tryptophan Metabolism
Pyridoxal 5 -Phosphate-Dependent Enzymes at the Crossroads of Host–Microbe Tryptophan Metabolism
Nicotinic Acid - an overview | ScienceDirect Topics
Niacin - Wikipedia
Nicotinamide - an overview | ScienceDirect Topics
Pyridoxal 5 -Phosphate-Dependent Enzymes at the Crossroads of Host–Microbe Tryptophan Metabolism
Tryptophan - an overview | ScienceDirect Topics
Tryptophan Biochemistry: Structural, Nutritional, Metabolic, and Medical Aspects in Humans
Nicotinic Acid - an overview | ScienceDirect Topics
What Is Tryptophan? Uses, Benefits, and Foods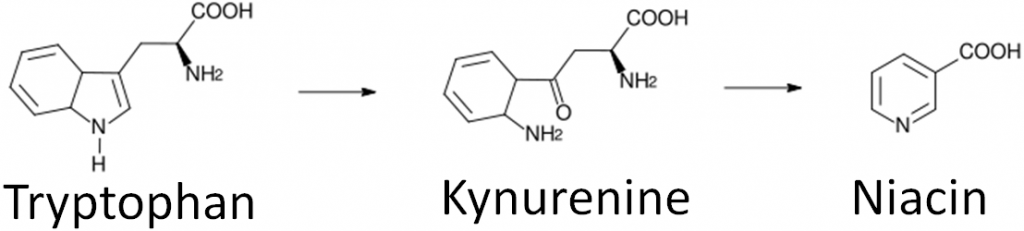
Macronutrient Metabolism Micronutrients – Human Nutrition
Phenylalanine - an overview | ScienceDirect Topics
Amino acid transporters revisited: New views in health and disease: Trends in Biochemical Sciences
Niacin - ScienceDirect
Nicotinic Acid - an overview | ScienceDirect Topics
Pyridoxal 5 -Phosphate-Dependent Enzymes at the Crossroads of Host–Microbe Tryptophan Metabolism
Pyridoxal 5 -Phosphate-Dependent Enzymes at the Crossroads of Host–Microbe Tryptophan Metabolism
Essential Amino Acids: Chart, Abbreviations and Structure | Technology Networks
vitamin B3 Archives | GEG Research and Consulting
Pyridoxamine Phosphate - an overview | ScienceDirect Topics
Essential Amino Acids: Chart, Abbreviations and Structure | Technology Networks
A Guide To 9 Essential Amino Acids & How To Get Them
What Is Tryptophan? Uses, Benefits, and Foods
Nicotinamide Derivative - an overview | ScienceDirect Topics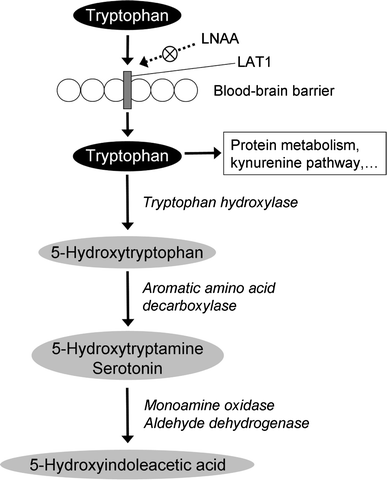
Tryptophan metabolism, from nutrition to potential therapeutic applications | SpringerLink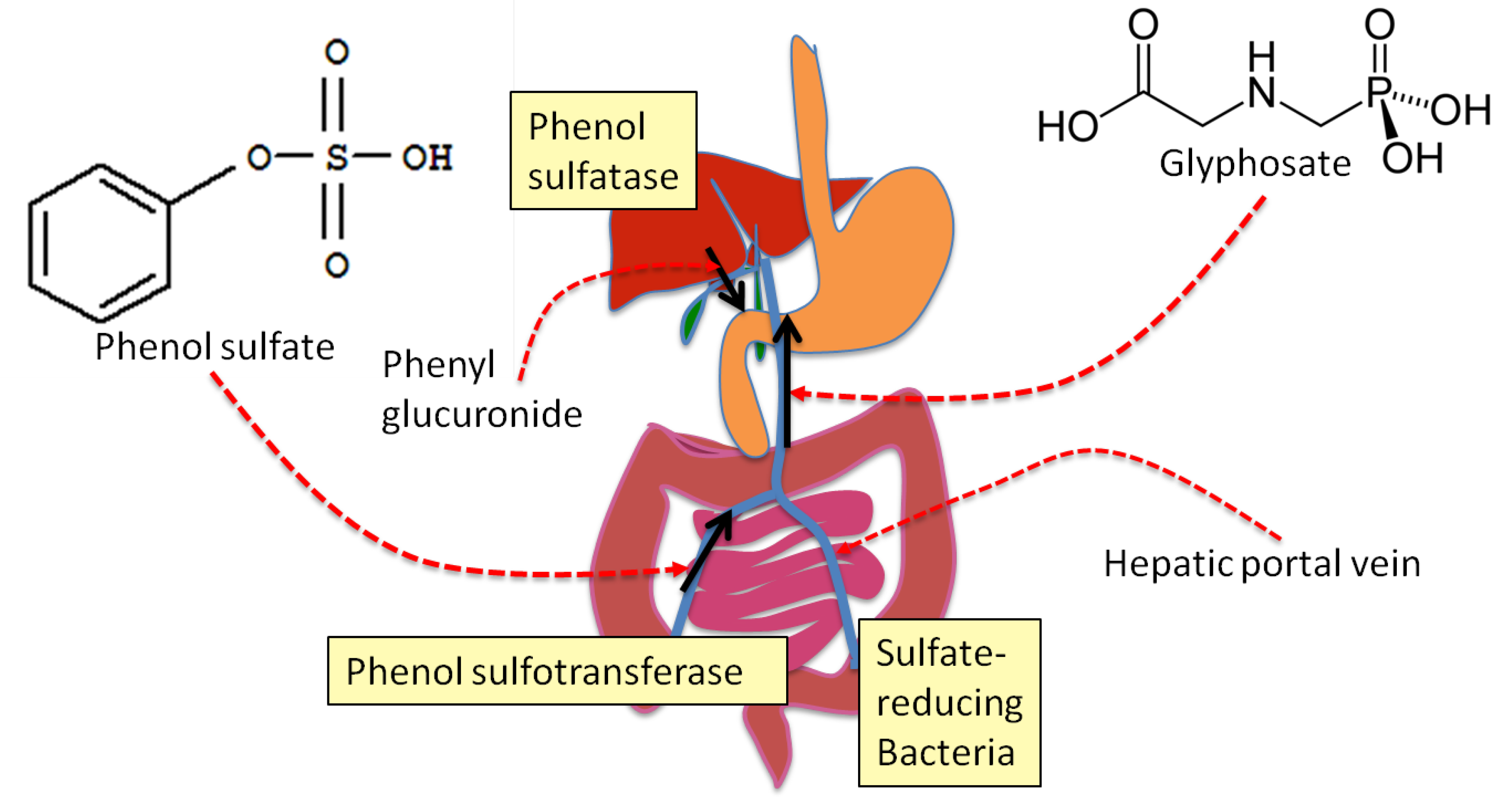
Entropy | Free Full-Text | Glyphosate's Suppression of Cytochrome P450 Enzymes and Amino Acid Biosynthesis by the Gut Microbiome: Pathways to Modern Diseases | HTML
Top 12 Foods That Are High in Vitamin B12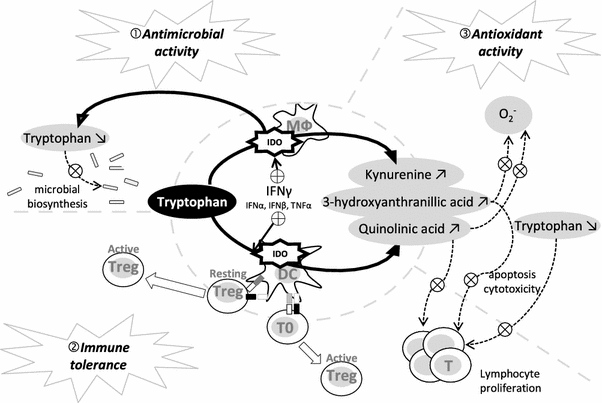
Tryptophan metabolism, from nutrition to potential therapeutic applications | SpringerLink
Top 12 Foods That Are High in Vitamin B12
PDF) Tryptophan and the immune response
Top 12 Foods That Are High in Vitamin B12
Vitamins - Block - - Major Reference Works - Wiley Online Library
STUDIES OF NIACIN REQUIREMENT IN MAN. I. EXPERIMENTAL PELLAGRA IN SUBJECTS ON CORN DIETS LOW IN NIACIN AND TRYPTOPHAN
 Nicotinamide - an overview | ScienceDirect Topics
Nicotinamide - an overview | ScienceDirect Topics
































Posting Komentar untuk "the fact that the amino acid tryptophan can be converted to niacin by the body explains why"